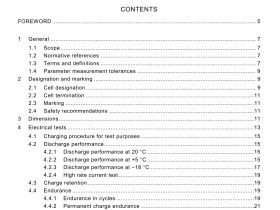
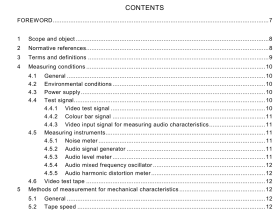
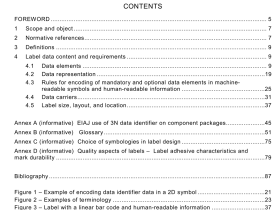
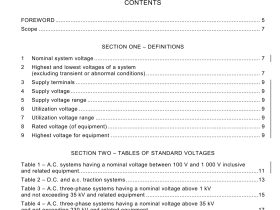
IEC 60601-2-34 pdf download
IEC 60601-2-34 pdf download.Medical electrical equipment
1 .1 Scope
Addition: This Particular Standard applies to INVASIVE BLOOD PRESSURE MONITORING and measuring equipment as defined in 2.1 01 , hereinafter referred to as EQUIPMENT . This Particular Standard does not apply to CATHETER tubing, CATHETER needles, Luer locks, taps and tap tables. This Particular Standard also does not apply to NON – INVASIVE BLOOD PRESSURE MONITORING EQUIPMENT . 1 .2 Object Replacement: The object of this Particular Standard is to establish particular requirements for the safety, including the essential performance of EQUIPMENT , as defined in 2.1 01 . 1.3 Particular Standards Addition: This Particular Standard refers to IEC 60601 -1 :1 988, Medical electrical equipment – Part 1: General requirements for safety as amended by its amendment 1 (1 991 ) and its amendment 2 (1 995). The General Standard takes into account IEC 60601 -1 -2:1 993, Medical electrical equipment – Part 1: General requirements for safety – 2. Collateral Standard: Electromagnetic compatibility – Requirements and tests and IEC 60601 -1 -4:1 996, Medical electrical equipment – Part 1: General requirements for safety – 4.Collateral Standard: Programmable electrical medical systems. For brevity, IEC 60601 is referred to in this Particular Standard either as the “General Standard” or as the “General Requirement(s)”.The numbering of sections, clauses or subclauses of this Particular Standard corresponds to that of the General Standard. The changes to the text of the General Standard are specified by the use of the following words: “Replacement” means that the clause or subclause of the General Standard is replaced completely by the text of this Particular Standard. “Addition” means that the clause or subclause of this Particular Standard is additional to the requirements of the General Standard. “Amendment” means that the clause or subclause of the General Standard is amended as indicated by the text of this Particular Standard. Subclauses or figures which are additional to those of the General Standard are numbered starting from 1 01 , additional annexes are lettered AA, BB, etc, and additional items aa), bb), etc. The term “this Standard” is used to make reference to the General Standard and this Particular Standard taken together. An asterisk (*) notes clauses for which there is rationale comment in Annex AA or Annex BB. It is considered that a knowledge of the reasons for these requirements will facilitate the proper application of the standard and be of use in any revision that may be necessitated by changes in clinical practice or as a result of developments in technology. Where there is no corresponding section, clause or subclause in this Particular Standard, the section, clause or subclause of the General Standard, although possibly not relevant, applies without modification; where it is intended that any part of the General Standard, although possibly relevant, is not to be applied, a statement to that effect is given in this Particular Standard. The requirements of this Particular Standard take priority over those of the General Standard and of the Collateral Standards mentioned above.
2 Terminology and definitions
This clause of the General Standard applies except as follows: 2.1.5 APPLIED PART Replacement: The TRANSDUCER , including any fluid-filled system Additional definitions: 2.1 01 INVASIVE BLOOD PRESSURE MONITORING EQUIPMENT ( EQUIPMENT ) stand-alone measuring equipment or part of a physiological monitoring or measuring system, including associated TRANSDUCERS , that is used for the internal measurement of circulatory system pressures 2.1 02 TRANSDUCER device for converting pressure into an electrical signal for monitoring or measuring 2.1 03 CATHETER TIP TRANSDUCER TRANSDUCER mounted at, or close to, the tip of a catheter and intended for insertion into the cardiovascular system 2.1 04 DOME means for hydraulically coupling the PATIENT ‘ S blood pressure to the TRANSDUCER , where a TRANSDUCER external to the PATIENT is used 2.1 2.101 ALARM signal which indicates abnormal events occurring to the PATIENT or EQUIPMENT 2.1 2.102 PHYSIOLOGICAL ALARM signal which either indicates that a monitored physiological parameter is out of specified limits or indicates an abnormal PATIENT condition 2.1 2.103 TECHNICAL ALARM signal which indicates that the EQUIPMENT or part(s) of the EQUIPMENT are not capable of accurately monitoring the PATIENT ‘ S condition 2.1 2.1 04 SILENCING stopping an auditory ALARM manifestation by manual action 2.1 2.105 SILENCING / RESET stopping a visual and/or auditory ALARM manifestation and reenabling system response to an abnormal PATIENT condition 2.1 2.106 INHIBITION disabling or SILENCING and disabling an ALARM until revoked intentionally 2.1 2.107 SUSPENSION disabling or SILENCING and disabling an ALARM temporarily